I will do the time-independent Schrödinger equation because it is easiest to understand without much mathematics. Also, I will do the one-dimensional version; think of a bead moving on a wire or perhaps a mass attached to a spring and bouncing back and forth.
First, do classical physics:
total energy E is the sum of kinetic
energy T (energy by virtue of motion)
plus potential energy V(x) (energy by virtue of position in some
force field), ![]() . The kinetic energy is
. The kinetic energy is ![]() where m is tha mass of the object and v is its speed. But, since linear
momentum p is
where m is tha mass of the object and v is its speed. But, since linear
momentum p is ![]() , kinetic energy is often written as
, kinetic energy is often written as ![]() , so
, so ![]() . Schrödinger’s equation is nothing more than the quantum
mechanical equivalent of this almost intuitive (the total is the sum of its
parts) energy equation.
. Schrödinger’s equation is nothing more than the quantum
mechanical equivalent of this almost intuitive (the total is the sum of its
parts) energy equation.
So, what is different about
quantum mechanics? It turns out that some quantities in nature must be treated
as mathematical operators, not as just simple numbers like they are in
classical mechanics. This is the case for linear momentum which becomes ![]() where
where
![]() , the imaginary unit,
, the imaginary unit, ![]() where h is a fundamental constant called
Planck’s constant, and
where h is a fundamental constant called
Planck’s constant, and ![]() is the derivative with
respect to x. If you have never
studied calculus, a derivative is an operator which, when it operates on a
mathematical function, tells you how fast that function is changing as x changes. Planck’s constant is an
extremely small number which is why quantum mechanics is not noticeable except
for very tiny systems (think atoms, nuclei, etc.).
is the derivative with
respect to x. If you have never
studied calculus, a derivative is an operator which, when it operates on a
mathematical function, tells you how fast that function is changing as x changes. Planck’s constant is an
extremely small number which is why quantum mechanics is not noticeable except
for very tiny systems (think atoms, nuclei, etc.).
Now, of necessity, things get a
little more mathematical; but if you get a little lost here, just carry on. We
still might want to know what the linear momentum is, not just what the
operator associated with it is. To do this we must introduce what is called an
eigenvalue equation which is of the form OΦ(x)=OΦ(x). O
is the operator, Φ(x) is called
the eigenfunction, and O is the called the eigenvalue. The
eigenfunction contains the information about what the system we are interested
in is, and the eigenvalue is what the observable quantity associated with the
operator is. For example, if it is linear momentum we are interested in, then ![]() where p is the value of the momentum you would
measure.
where p is the value of the momentum you would
measure.
Back to Schrödinger’s equation:
we still use ![]() but now
but now
![]() . (
. (![]() means
the derivative of the derivative, but that is not so important, just math.) So
now the total energy of a system is an operator, not a number. This operator is
called the Hamiltonian,
means
the derivative of the derivative, but that is not so important, just math.) So
now the total energy of a system is an operator, not a number. This operator is
called the Hamiltonian, ![]() . To find the energy of a system, therefore, there must be an
eigenvalue equation; this is Schrödinger’s equation
. To find the energy of a system, therefore, there must be an
eigenvalue equation; this is Schrödinger’s equation ![]() . The eigenfunction
. The eigenfunction ![]() is called the
wavefunction and has a very special meaning. It has no particular physical
meaning on its own but, when squared, it becomes the
probability density function. This means that
is called the
wavefunction and has a very special meaning. It has no particular physical
meaning on its own but, when squared, it becomes the
probability density function. This means that ![]() is the probability of
finding the particle between the positions x
and x+dx where dx is some very small change in x.
Of course, if
is the probability of
finding the particle between the positions x
and x+dx where dx is some very small change in x.
Of course, if ![]() is to have this
meaning, the sum of all probabilities over all space must add up to 1 ( the particle has to be somewhere).
is to have this
meaning, the sum of all probabilities over all space must add up to 1 ( the particle has to be somewhere).
From here on it is a matter of
applying this equation to a particular system to find out what energies will
satisfy the equation. A one-dimensional example is a mass on a spring. When you
put in the appropriate potential energy function you find that the spring
cannot have just any old energy, there are only certain energies which will
satisfy the Schrödinger equation; these are ![]() where n is any positive integer or zero and f is the frequency which the particular
spring oscillates with. Another example is an atom. Here, of course, we have a
three-dimensional problem and have to use a three-dimensional Schrödinger
equation which would have the form
where n is any positive integer or zero and f is the frequency which the particular
spring oscillates with. Another example is an atom. Here, of course, we have a
three-dimensional problem and have to use a three-dimensional Schrödinger
equation which would have the form 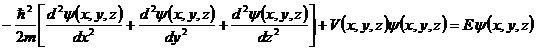 . What you find is still that an atom can only exist in
certain energy states, not any old energy you might want.
. What you find is still that an atom can only exist in
certain energy states, not any old energy you might want.